Eosinophilic Esophagitis Explained: Unraveling the Complexities of a Rapidly Emerging Esophageal Disorder. Discover the Latest Insights, Treatments, and What the Future Holds. (2025)
- Introduction: Defining Eosinophilic Esophagitis (EoE)
- Epidemiology and Rising Prevalence: A 15% Annual Increase in Diagnoses
- Pathophysiology: Immune Mechanisms and Genetic Factors
- Clinical Presentation: Symptoms Across Age Groups
- Diagnostic Criteria and Advances in Endoscopic Techniques
- Current Treatment Strategies: Diet, Medication, and Emerging Therapies
- Impact on Quality of Life and Long-Term Complications
- Technological Innovations: Non-Invasive Monitoring and Biomarkers
- Public Awareness, Advocacy, and Patient Support Resources
- Future Outlook: Research Directions and Forecasting EoE’s Public Health Impact
- Sources & References
Introduction: Defining Eosinophilic Esophagitis (EoE)
Eosinophilic Esophagitis (EoE) is a chronic, immune-mediated inflammatory disease of the esophagus characterized by eosinophil-predominant infiltration of the esophageal mucosa. First recognized as a distinct clinical entity in the 1990s, EoE has since emerged as a significant cause of esophageal dysfunction, particularly in children and young adults. The disease manifests with symptoms such as dysphagia (difficulty swallowing), food impaction, and, in pediatric populations, feeding difficulties and failure to thrive. Histologically, EoE is defined by the presence of at least 15 eosinophils per high-power field in esophageal biopsies, in the absence of other causes of esophageal eosinophilia.
The pathogenesis of EoE is complex, involving genetic predisposition, environmental exposures, and immune dysregulation, particularly in response to food and aeroallergens. The disease is now recognized as part of the spectrum of atopic disorders, frequently co-occurring with conditions such as asthma, allergic rhinitis, and atopic dermatitis. The prevalence of EoE has increased markedly over the past two decades, with recent epidemiological studies indicating a continued rise in incidence and recognition worldwide. Current estimates suggest a prevalence of approximately 1 in 2,000 individuals in Western countries, though rates may vary by region and population studied.
Diagnosis of EoE relies on a combination of clinical presentation, endoscopic findings (such as esophageal rings, furrows, and exudates), and histopathological confirmation. The disease is often underdiagnosed or misdiagnosed due to symptom overlap with gastroesophageal reflux disease (GERD) and other esophageal disorders. In 2025, the diagnostic criteria and management guidelines for EoE continue to be refined by leading organizations such as the American Gastroenterological Association and the European Academy of Allergy and Clinical Immunology, reflecting advances in understanding of disease mechanisms and therapeutic options.
Looking ahead, the outlook for EoE is shaped by ongoing research into its immunopathology, the development of targeted biologic therapies, and efforts to improve early diagnosis and patient quality of life. As awareness grows among healthcare providers and the public, and as new treatments become available, the management of EoE is expected to become increasingly personalized and effective in the coming years.
Epidemiology and Rising Prevalence: A 15% Annual Increase in Diagnoses
Eosinophilic esophagitis (EoE) has emerged as a significant and increasingly recognized chronic immune-mediated disease of the esophagus, characterized by eosinophil-predominant inflammation and symptoms of esophageal dysfunction. Over the past decade, and particularly into 2025, epidemiological data indicate a striking rise in the prevalence and incidence of EoE worldwide. Recent analyses suggest that the annual rate of new EoE diagnoses is increasing by approximately 15%, a trend observed across North America, Europe, and parts of Asia.
This surge is attributed to a combination of heightened clinical awareness, improved diagnostic protocols, and possibly genuine increases in disease occurrence. The widespread adoption of endoscopic biopsy protocols and updated histopathological criteria have enabled more accurate and earlier detection of EoE, contributing to the observed rise in case numbers. For instance, the Centers for Disease Control and Prevention (CDC) in the United States and the National Health Service (NHS) in the United Kingdom have both reported a steady uptick in EoE diagnoses, particularly among children and young adults.
Epidemiological studies published in recent years estimate the current prevalence of EoE in Western countries to be between 1 in 1,000 and 1 in 2,000 individuals, with some regions reporting even higher rates. The disease is more common in males, with a male-to-female ratio of approximately 3:1, and is frequently associated with other atopic conditions such as asthma, allergic rhinitis, and food allergies. The World Allergy Organization, a leading global authority on allergic diseases, has highlighted EoE as a growing public health concern, emphasizing the need for increased research and awareness.
Looking ahead to the next few years, experts anticipate that the prevalence of EoE will continue to rise, driven by ongoing improvements in diagnostic sensitivity and possibly by environmental or lifestyle factors that remain under investigation. The increasing burden of EoE is expected to place additional demands on healthcare systems, particularly in pediatric gastroenterology and allergy services. In response, organizations such as the National Institute of Allergy and Infectious Diseases (NIAID) are prioritizing research into the underlying causes, risk factors, and optimal management strategies for EoE.
In summary, the epidemiology of eosinophilic esophagitis in 2025 is marked by a notable and ongoing rise in diagnoses, with a 15% annual increase reflecting both improved recognition and a possible true increase in disease incidence. Continued surveillance, research, and public health initiatives will be essential to address the growing impact of EoE in the coming years.
Pathophysiology: Immune Mechanisms and Genetic Factors
Eosinophilic Esophagitis (EoE) is a chronic, immune-mediated disease characterized by eosinophil-predominant inflammation of the esophagus. The pathophysiology of EoE is complex, involving both immune mechanisms and genetic predispositions. As of 2025, research continues to elucidate the interplay between environmental triggers, immune responses, and genetic factors that drive the disease process.
The immune response in EoE is primarily Th2-mediated, with interleukin-5 (IL-5), interleukin-13 (IL-13), and eotaxin-3 playing central roles. These cytokines promote the recruitment and activation of eosinophils in the esophageal mucosa. Recent studies have highlighted the importance of epithelial barrier dysfunction, which allows allergens—often food proteins—to penetrate and trigger local immune activation. This leads to chronic inflammation, tissue remodeling, and, ultimately, esophageal dysfunction.
Genetic susceptibility is increasingly recognized as a key factor in EoE. Genome-wide association studies (GWAS) have identified several risk loci, including variants in the thymic stromal lymphopoietin (TSLP) gene and the eotaxin-3 (CCL26) gene. These genes are involved in the regulation of immune responses and eosinophil trafficking. Ongoing research in 2025 is focusing on the functional consequences of these variants, with the aim of identifying potential therapeutic targets.
Epigenetic modifications and gene-environment interactions are also under investigation. For example, environmental factors such as early-life antibiotic exposure, cesarean delivery, and changes in the microbiome have been associated with increased risk of EoE. These factors may influence gene expression and immune system development, further predisposing individuals to the disease.
In the next few years, advances in single-cell RNA sequencing and spatial transcriptomics are expected to provide deeper insights into the cellular and molecular landscape of EoE. These technologies will help delineate the specific immune cell populations and signaling pathways involved in disease initiation and progression. Additionally, the identification of biomarkers for disease activity and response to therapy remains a major research priority.
Key organizations such as the National Institutes of Health and the American Academy of Allergy, Asthma & Immunology are supporting research initiatives aimed at unraveling the pathophysiology of EoE. Their efforts are expected to accelerate the development of targeted therapies and improve diagnostic strategies, ultimately enhancing patient outcomes in the coming years.
Clinical Presentation: Symptoms Across Age Groups
Eosinophilic Esophagitis (EoE) is a chronic, immune-mediated esophageal disease characterized by symptoms related to esophageal dysfunction and histologically by eosinophil-predominant inflammation. The clinical presentation of EoE varies significantly across age groups, reflecting both developmental differences in symptom expression and evolving diagnostic awareness. As of 2025, increased recognition and improved diagnostic criteria have led to earlier and more accurate identification of EoE in both pediatric and adult populations.
In infants and young children, EoE often presents with non-specific symptoms such as feeding difficulties, failure to thrive, vomiting, and abdominal pain. These symptoms can be subtle and are frequently mistaken for more common pediatric conditions like gastroesophageal reflux disease (GERD) or food allergies. School-aged children may report dysphagia (difficulty swallowing), food impaction, and persistent abdominal discomfort. Behavioral adaptations, such as prolonged mealtimes, excessive chewing, or avoidance of certain food textures, are increasingly recognized as indirect indicators of EoE in this age group. Recent clinical guidelines emphasize the importance of considering EoE in children with refractory feeding issues or unexplained gastrointestinal symptoms, leading to a rise in pediatric diagnoses in the past few years (National Institute of Allergy and Infectious Diseases).
In adolescents and adults, the clinical picture shifts toward more classic esophageal symptoms. Dysphagia is the most common complaint, often accompanied by episodes of food impaction that may require emergency intervention. Retrospective studies and registry data from 2023–2025 indicate that up to 70% of adults with EoE report a history of food impaction, and many describe a long-standing pattern of adapting their eating habits to avoid symptoms. Chest pain, heartburn, and upper abdominal pain are also reported, but these are less specific and can overlap with other esophageal disorders. Notably, the chronicity of symptoms in adults often leads to esophageal remodeling and strictures, which can complicate management if diagnosis is delayed (American Gastroenterological Association).
The outlook for 2025 and beyond includes ongoing efforts to refine symptom-based screening tools and increase awareness among primary care providers and specialists. Multicenter studies are underway to better characterize age-specific symptom profiles and to validate non-invasive diagnostic markers, aiming to reduce the time from symptom onset to diagnosis. As understanding of EoE’s natural history improves, clinicians are increasingly able to tailor management strategies to the patient’s age and symptom burden, improving quality of life and long-term outcomes (National Institute of Allergy and Infectious Diseases).
Diagnostic Criteria and Advances in Endoscopic Techniques
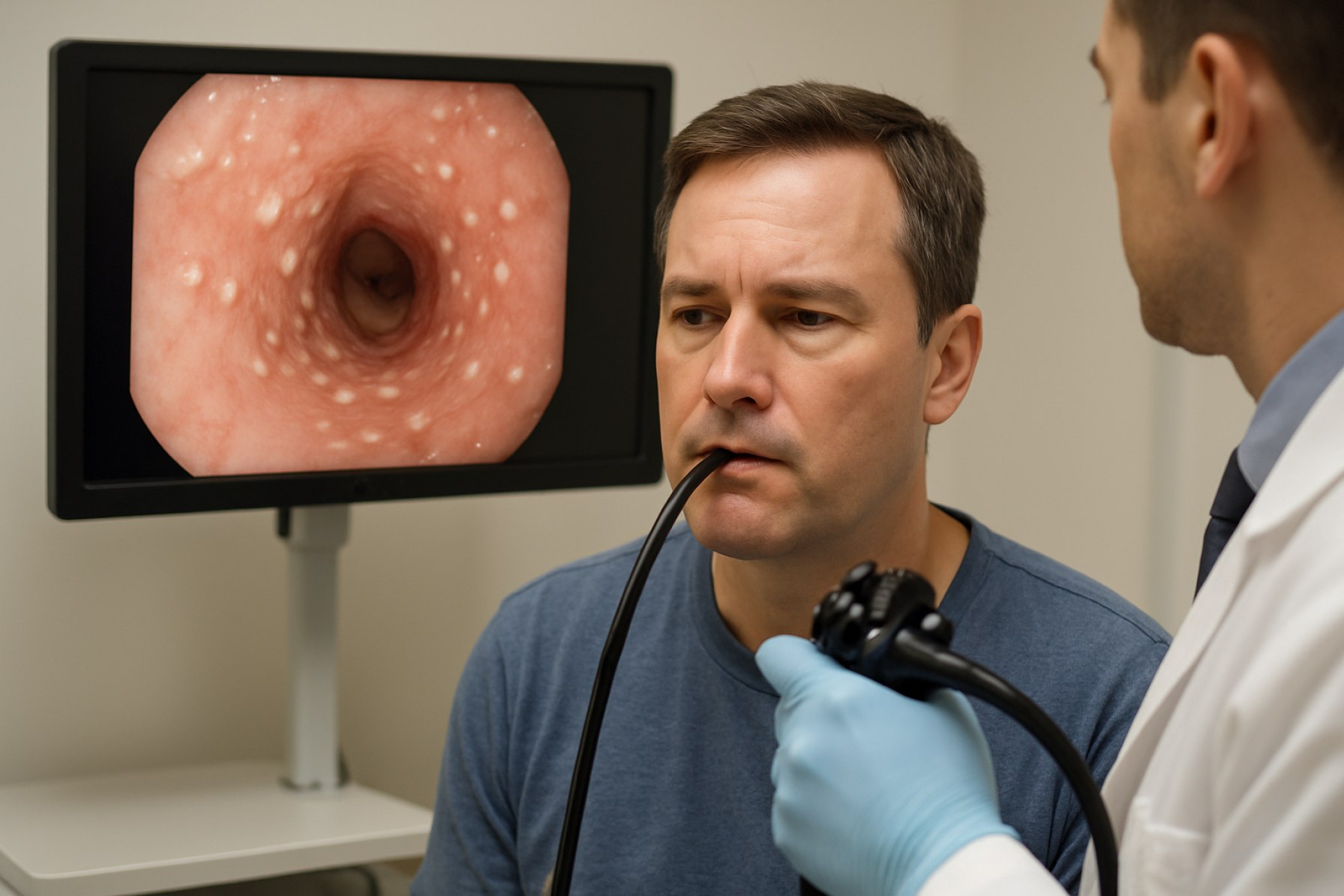
Eosinophilic Esophagitis (EoE) is a chronic, immune-mediated esophageal disease characterized by symptoms of esophageal dysfunction and histologically by eosinophil-predominant inflammation. Diagnostic criteria for EoE have evolved significantly, with recent years seeing a refinement in both clinical and histopathological standards. As of 2025, the diagnosis of EoE requires the presence of symptoms related to esophageal dysfunction, a peak eosinophil count of ≥15 eosinophils per high-power field (eos/hpf) on esophageal biopsy, and exclusion of other causes of esophageal eosinophilia, such as gastroesophageal reflux disease (GERD) and infections. The consensus guidelines, regularly updated by international gastroenterology societies, emphasize the importance of integrating clinical, endoscopic, and histological findings for accurate diagnosis (American Gastroenterological Association).
Endoscopic evaluation remains a cornerstone in the diagnosis and management of EoE. Traditional endoscopic findings include rings (trachealization), linear furrows, white exudates, and strictures. However, these features can be subtle or absent, especially in early disease. In response, the EoE Endoscopic Reference Score (EREFS) has been widely adopted to standardize the assessment of endoscopic features, improving interobserver reliability and facilitating longitudinal disease monitoring. The EREFS system is now routinely used in both clinical practice and research settings (American Society for Gastrointestinal Endoscopy).
Recent advances in endoscopic techniques are poised to further enhance the diagnosis and management of EoE. High-resolution endoscopy and image-enhanced modalities, such as narrow-band imaging (NBI) and confocal laser endomicroscopy, are being increasingly utilized to improve visualization of subtle mucosal changes and guide targeted biopsies. These technologies have demonstrated improved sensitivity in detecting EoE-related changes, potentially reducing the need for multiple random biopsies. Additionally, non-invasive and minimally invasive diagnostic tools, such as the esophageal string test and cytosponge, are under active investigation and may soon complement or partially replace traditional endoscopic biopsy, especially for disease monitoring (National Institutes of Health).
Looking ahead, the integration of artificial intelligence (AI) into endoscopic image analysis is expected to further refine the detection and characterization of EoE. Early studies suggest that AI-assisted endoscopy can improve diagnostic accuracy and reduce interobserver variability. As these technologies mature and become more widely available, they are likely to be incorporated into routine clinical workflows within the next few years, supporting earlier diagnosis and more personalized management of EoE.
Current Treatment Strategies: Diet, Medication, and Emerging Therapies
Eosinophilic Esophagitis (EoE) is a chronic, immune-mediated esophageal disease characterized by eosinophil-predominant inflammation and symptoms of esophageal dysfunction. As of 2025, treatment strategies for EoE continue to evolve, with a focus on dietary management, pharmacologic interventions, and the development of novel therapies.
Dietary Management remains a cornerstone of EoE therapy. The empiric six-food elimination diet (SFED), which removes milk, wheat, eggs, soy, nuts, and seafood, has demonstrated efficacy in inducing histologic remission in both pediatric and adult populations. Recent years have seen a trend toward less restrictive approaches, such as the four-food or two-food elimination diets, aiming to balance efficacy with patient quality of life. Elemental diets, consisting of amino acid-based formulas, are highly effective but often reserved for refractory cases due to palatability and cost concerns. Ongoing research is refining reintroduction protocols and identifying biomarkers to predict dietary responders, with the goal of personalizing dietary therapy.
Pharmacologic Therapy is primarily centered on topical corticosteroids, such as fluticasone and budesonide, which are swallowed rather than inhaled. These agents remain first-line pharmacologic options, with multiple studies confirming their efficacy in reducing esophageal eosinophilia and improving symptoms. In 2022, the U.S. Food and Drug Administration (FDA) approved budesonide oral suspension specifically for EoE, marking a significant milestone in disease-specific drug development. Proton pump inhibitors (PPIs) are also widely used, as they can induce remission in a subset of patients, likely due to both acid suppression and anti-inflammatory effects. The optimal duration and dosing of these therapies continue to be areas of active investigation.
Emerging Therapies are rapidly advancing, particularly with the advent of biologic agents targeting key inflammatory pathways. Dupilumab, a monoclonal antibody that inhibits interleukin-4 and interleukin-13 signaling, received FDA approval for EoE in 2022, representing the first biologic therapy for this indication. Clinical trials are ongoing for other biologics, such as anti-IL-5 and anti-Siglec-8 agents, which may offer additional options for patients with refractory disease. The pipeline for EoE therapeutics is robust, with several agents in phase II and III trials as of 2025.
Looking ahead, the outlook for EoE management is promising. The integration of precision medicine, including genetic and molecular profiling, is expected to refine treatment selection and improve outcomes. Multidisciplinary care involving gastroenterologists, allergists, dietitians, and patient advocacy organizations is increasingly recognized as essential for optimal management. Continued collaboration among stakeholders, including regulatory agencies such as the U.S. Food and Drug Administration and research consortia like the National Institutes of Health, will be critical in advancing therapeutic options and improving quality of life for individuals with EoE.
Impact on Quality of Life and Long-Term Complications
Eosinophilic Esophagitis (EoE) is a chronic, immune-mediated esophageal disease that significantly impacts patients’ quality of life and carries risks for long-term complications. As of 2025, the burden of EoE is increasingly recognized by clinicians and patient advocacy groups, with ongoing research highlighting both the physical and psychosocial dimensions of the disease.
Patients with EoE often experience persistent symptoms such as dysphagia (difficulty swallowing), food impaction, chest pain, and, in children, feeding difficulties and failure to thrive. These symptoms can lead to anxiety around eating, social withdrawal, and reduced participation in daily activities. Recent patient-reported outcome studies have shown that adults and children with EoE report lower health-related quality of life compared to the general population, with particular challenges in social functioning and emotional well-being. The chronic nature of EoE, frequent need for endoscopic procedures, and dietary restrictions further contribute to psychological stress and decreased life satisfaction.
Long-term complications of EoE are increasingly documented. Chronic inflammation of the esophagus can lead to tissue remodeling, resulting in esophageal strictures (narrowing) and rings, which further exacerbate swallowing difficulties and increase the risk of food impaction. Data from longitudinal cohorts indicate that, without effective management, up to 30-50% of adults with EoE may develop strictures over time. Pediatric patients are also at risk for growth impairment and nutritional deficiencies due to restricted diets and feeding aversion.
The outlook for 2025 and the coming years is shaped by advances in both awareness and therapeutic options. The approval of the first biologic therapy for EoE in 2022 marked a significant milestone, and ongoing clinical trials are evaluating additional targeted treatments that may reduce inflammation and prevent long-term complications. Organizations such as the U.S. Food and Drug Administration and the National Institutes of Health are supporting research into the pathophysiology and management of EoE, while patient advocacy groups like the American Partnership for Eosinophilic Disorders are working to improve patient education and access to care.
- Quality of life remains a central concern, with multidisciplinary care—including gastroenterologists, allergists, dietitians, and mental health professionals—recommended for optimal management.
- Early diagnosis and intervention are critical to prevent irreversible esophageal damage and improve long-term outcomes.
- Ongoing research and new therapies are expected to further reduce the burden of disease and improve quality of life for patients with EoE in the near future.
Technological Innovations: Non-Invasive Monitoring and Biomarkers
Technological innovation in the monitoring and diagnosis of Eosinophilic Esophagitis (EoE) is accelerating, with a strong focus on non-invasive methods and biomarker development. Traditionally, EoE diagnosis and monitoring have relied on repeated endoscopies with esophageal biopsies, which are invasive, costly, and burdensome for patients. In 2025 and the coming years, several promising technologies and approaches are poised to transform clinical practice.
One of the most significant advances is the refinement and broader adoption of the esophageal string test (EST) and the Cytosponge. These minimally invasive devices collect esophageal samples for analysis of eosinophil-associated proteins and other biomarkers, reducing the need for endoscopy. The National Institutes of Health and leading academic centers have supported multicenter studies demonstrating the EST’s accuracy in monitoring disease activity and response to therapy. The Cytosponge, already used in Barrett’s esophagus screening, is being adapted for EoE, with ongoing trials evaluating its sensitivity and specificity for detecting eosinophilic inflammation.
Salivary and blood-based biomarkers are also under intense investigation. Recent research has identified panels of cytokines, chemokines, and microRNAs in blood and saliva that correlate with esophageal eosinophilia and disease activity. The U.S. Food and Drug Administration has granted breakthrough device designation to several companies developing multiplex assays for EoE biomarkers, expediting their path to clinical use. These assays aim to provide real-time, non-invasive monitoring, enabling more personalized and timely adjustments to therapy.
Advances in imaging technologies, such as high-resolution impedance planimetry (EndoFLIP) and confocal laser endomicroscopy, are also contributing to less invasive assessment of esophageal function and structure. While these modalities still require endoscopic access, ongoing miniaturization and integration with non-endoscopic platforms are expected to make them more accessible in outpatient settings within the next few years.
Looking ahead, the integration of artificial intelligence (AI) and machine learning with biomarker data and imaging holds promise for further improving diagnostic accuracy and predicting disease progression. Collaborative efforts by organizations such as the American Academy of Allergy, Asthma & Immunology and the American Gastroenterological Association are driving the development of standardized protocols and validation studies, which are essential for regulatory approval and widespread adoption.
In summary, 2025 marks a pivotal year for non-invasive EoE monitoring, with technological innovations set to reduce patient burden, enhance disease management, and enable precision medicine approaches in the near future.
Public Awareness, Advocacy, and Patient Support Resources
Public awareness and advocacy for Eosinophilic Esophagitis (EoE) have grown significantly in recent years, with 2025 marking a period of increased visibility and resource development for patients and families. EoE, a chronic, immune-mediated esophageal disease, has historically been underrecognized, but ongoing efforts by patient organizations, medical societies, and governmental agencies are changing this landscape.
Key organizations such as the American Partnership for Eosinophilic Disorders (APFED) and the Cincinnati Children's Hospital Medical Center continue to play pivotal roles in advocacy, education, and support. APFED, a leading nonprofit, has expanded its outreach in 2025 through national awareness campaigns, educational webinars, and the distribution of updated patient toolkits. These resources are designed to help patients navigate diagnosis, dietary management, and treatment options, as well as to foster connections within the EoE community.
Medical societies such as the American Gastroenterological Association (AGA) and the American Academy of Allergy, Asthma & Immunology (AAAAI) have also increased their focus on EoE. In 2025, these organizations are providing updated clinical guidelines, hosting professional education sessions, and supporting research initiatives aimed at improving patient outcomes. Their efforts contribute to greater awareness among healthcare providers, which is critical for early diagnosis and effective management.
Governmental agencies, including the National Institutes of Health (NIH), have recognized EoE as a significant public health concern. The NIH continues to fund research and support public information campaigns, helping to disseminate accurate, evidence-based information to both clinicians and the public. In 2025, the NIH is also supporting collaborative projects that bring together researchers, clinicians, and patient advocates to address gaps in care and knowledge.
Looking ahead, the outlook for public awareness and patient support in EoE is positive. Digital platforms and social media are being leveraged to reach broader audiences, while virtual support groups and telehealth resources are making it easier for patients in remote areas to access care and community. The continued collaboration between advocacy groups, medical societies, and governmental agencies is expected to further enhance education, reduce stigma, and improve quality of life for those affected by EoE in the coming years.
Future Outlook: Research Directions and Forecasting EoE’s Public Health Impact
Eosinophilic Esophagitis (EoE) is increasingly recognized as a significant chronic immune-mediated disease, with its prevalence rising globally. As of 2025, research and public health initiatives are intensifying to address the growing burden of EoE, focusing on improved diagnostics, novel therapeutics, and a deeper understanding of its epidemiology and long-term impact.
Current research directions are heavily influenced by advances in molecular biology and immunology. Investigators are exploring the genetic and environmental factors contributing to EoE, with large-scale genomic studies underway to identify susceptibility loci and potential biomarkers for early detection and personalized treatment. The role of the esophageal microbiome and its interaction with dietary antigens is also a key area of investigation, aiming to clarify pathogenesis and identify new therapeutic targets.
Therapeutic innovation is a major focus for 2025 and beyond. The recent approval of biologic agents, such as monoclonal antibodies targeting interleukin pathways, marks a paradigm shift in EoE management. Ongoing clinical trials are evaluating the long-term efficacy and safety of these agents, as well as their impact on disease remission and quality of life. Additionally, non-invasive diagnostic tools, including minimally invasive esophageal sampling devices and advanced imaging techniques, are being developed to reduce reliance on endoscopy and improve patient monitoring.
From a public health perspective, the increasing incidence of EoE—particularly in children and young adults—poses challenges for healthcare systems. Forecasts suggest that the prevalence will continue to rise over the next decade, driven by heightened awareness, improved diagnostic criteria, and possible environmental factors. This trend underscores the need for updated clinical guidelines, expanded provider education, and patient support resources.
Organizations such as the National Institutes of Health and the U.S. Food and Drug Administration are supporting research and regulatory pathways for new therapies, while patient advocacy groups are playing a crucial role in raising awareness and funding research. Internationally, collaborative efforts are underway to harmonize diagnostic standards and share epidemiological data, as seen in initiatives led by the World Health Organization.
Looking ahead, the outlook for EoE research and public health impact is one of cautious optimism. Continued investment in translational research, coupled with coordinated public health strategies, is expected to yield more effective treatments, earlier diagnosis, and ultimately, improved outcomes for individuals affected by EoE in the coming years.
Sources & References
- European Academy of Allergy and Clinical Immunology
- Centers for Disease Control and Prevention
- National Health Service
- World Allergy Organization
- National Institute of Allergy and Infectious Diseases
- National Institutes of Health
- American Academy of Allergy, Asthma & Immunology
- American Society for Gastrointestinal Endoscopy
- National Institutes of Health
- American Partnership for Eosinophilic Disorders
- American Academy of Allergy, Asthma & Immunology
- Cincinnati Children's Hospital Medical Center
- World Health Organization